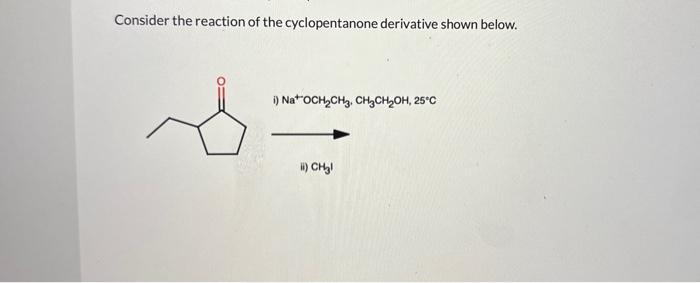
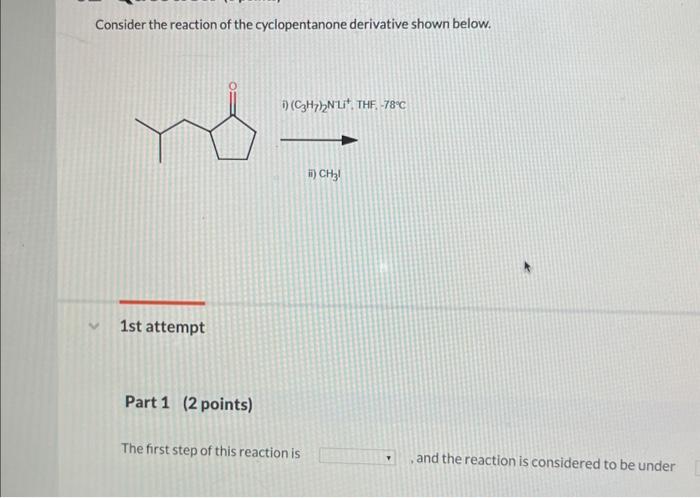
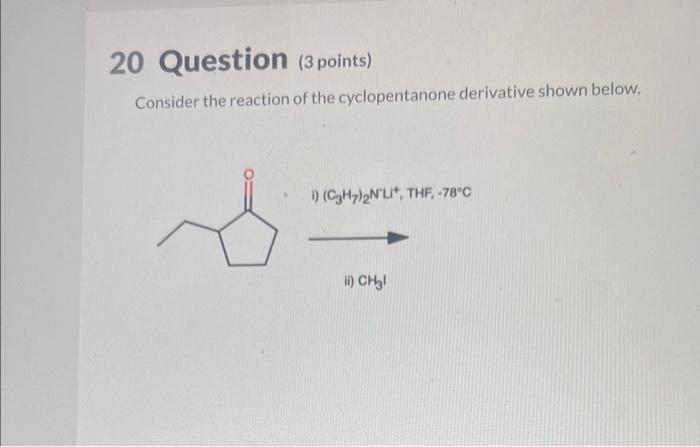
Consider the reaction of the cyclopentanone derivative shown below. – Consider the reaction of the cyclopentanone derivative shown below, and embark on a journey into the realm of organic chemistry. This discourse delves into the intricate structural features, reactivity patterns, and synthetic applications of cyclopentanone derivatives, providing a comprehensive understanding of their significance in modern chemistry.
Cyclopentanone derivatives, characterized by their cyclic structure and diverse substituents, exhibit unique reactivity towards nucleophilic addition reactions. The regio- and stereoselective nature of these reactions, influenced by both the cyclopentanone derivative and the nucleophile, offers a fascinating insight into the factors governing chemical transformations.
Structural Features of Cyclopentanone Derivatives: Consider The Reaction Of The Cyclopentanone Derivative Shown Below.
Cyclopentanone derivatives are cyclic compounds containing a five-membered cyclopentanone ring. The ring may have various substituents, including alkyl, alkenyl, aryl, or heteroaryl groups. The stereochemistry of the cyclopentanone ring can be either cis or trans.
Reactivity of Cyclopentanone Derivatives
Cyclopentanone derivatives are highly reactive towards nucleophilic addition reactions. The carbonyl group of the cyclopentanone ring acts as an electrophile and can be attacked by a variety of nucleophiles, such as Grignard reagents, organolithium compounds, and alkoxides.
The mechanism of nucleophilic addition to cyclopentanone derivatives involves the initial formation of a tetrahedral intermediate. This intermediate then collapses to form a new carbon-carbon bond and an alkoxide ion.
Examples of nucleophilic addition reactions involving cyclopentanone derivatives include the addition of Grignard reagents to form alcohols, the addition of organolithium compounds to form ketones, and the addition of alkoxides to form acetals.
Regio- and Stereoselectivity of Nucleophilic Addition Reactions, Consider the reaction of the cyclopentanone derivative shown below.
The regio- and stereoselectivity of nucleophilic addition reactions to cyclopentanone derivatives are influenced by a number of factors, including the structure of the cyclopentanone derivative, the structure of the nucleophile, and the reaction conditions.
The structure of the cyclopentanone derivative can affect the regioselectivity of the reaction. For example, if the cyclopentanone ring is substituted with an electron-withdrawing group, the nucleophile will be more likely to add to the carbon atom adjacent to the electron-withdrawing group.
The structure of the nucleophile can also affect the regio- and stereoselectivity of the reaction. For example, if the nucleophile is a bulky nucleophile, it will be more likely to add to the less substituted carbon atom of the cyclopentanone ring.
The reaction conditions can also affect the regio- and stereoselectivity of the reaction. For example, if the reaction is carried out in a polar solvent, the nucleophile will be more likely to add to the more polar carbon atom of the cyclopentanone ring.
Synthetic Applications of Cyclopentanone Derivatives
Cyclopentanone derivatives are versatile starting materials and intermediates in organic chemistry. They can be used to synthesize a variety of compounds, including alcohols, ketones, aldehydes, and carboxylic acids.
One of the most common uses of cyclopentanone derivatives is in the synthesis of alcohols. Cyclopentanone derivatives can be reduced to alcohols using a variety of reducing agents, such as sodium borohydride, lithium aluminum hydride, and diisobutylaluminum hydride.
Cyclopentanone derivatives can also be used to synthesize ketones. Ketones can be prepared by the oxidation of cyclopentanone derivatives using a variety of oxidizing agents, such as potassium permanganate, chromium trioxide, and manganese dioxide.
FAQ Section
What is the significance of the cyclic structure in cyclopentanone derivatives?
The cyclic structure imparts rigidity and conformational stability, influencing the reactivity and selectivity of the cyclopentanone derivative.
How does the stereochemistry of the cyclopentanone derivative affect its reactivity?
Stereochemistry dictates the spatial arrangement of substituents, which can influence the accessibility and reactivity of the cyclopentanone derivative towards nucleophilic addition.
What factors govern the regio- and stereoselectivity of nucleophilic addition reactions?
Electronic effects, steric hindrance, and the nature of the nucleophile all play crucial roles in determining the regio- and stereochemical outcome of nucleophilic addition reactions.